Dipole Interaction Smaller differences require a stronger dipole phase GroupPresentation Title Agilent Restricted Page 28 C181 Methyl trans-9-octadecenoate C181 Methyl cis-9-octadecenoate BPt. Is CO2 a dipole-dipole.
Which Of The Following Compounds Exhibits Only Dispersion And Dipole Dipole Intermolecular Interactions A Hbr B Co2 C H2o D N2 Socratic
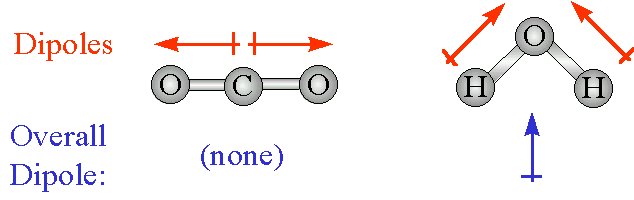
A molecule like CO2 may be composed of two dipoles but it has no dipole moment.

. Learn vocabulary terms and more with flashcards games and other study tools. Using photoelectron imaging and computational chemistry we show that photoexcitation by UVA light leads to the formation of CO2 CO and CH3. The observation of the unusual methide anion.
The common types of intermolecular forces of attraction that may exist for compounds such as methanol are hydrogen bonding London Dispersion Force or the dipole-dipole force of attraction. CO2 is a linear molecule so our dipoles are.

Solved 1 Point What Is The Strongest Intermolecular Force Chegg Com
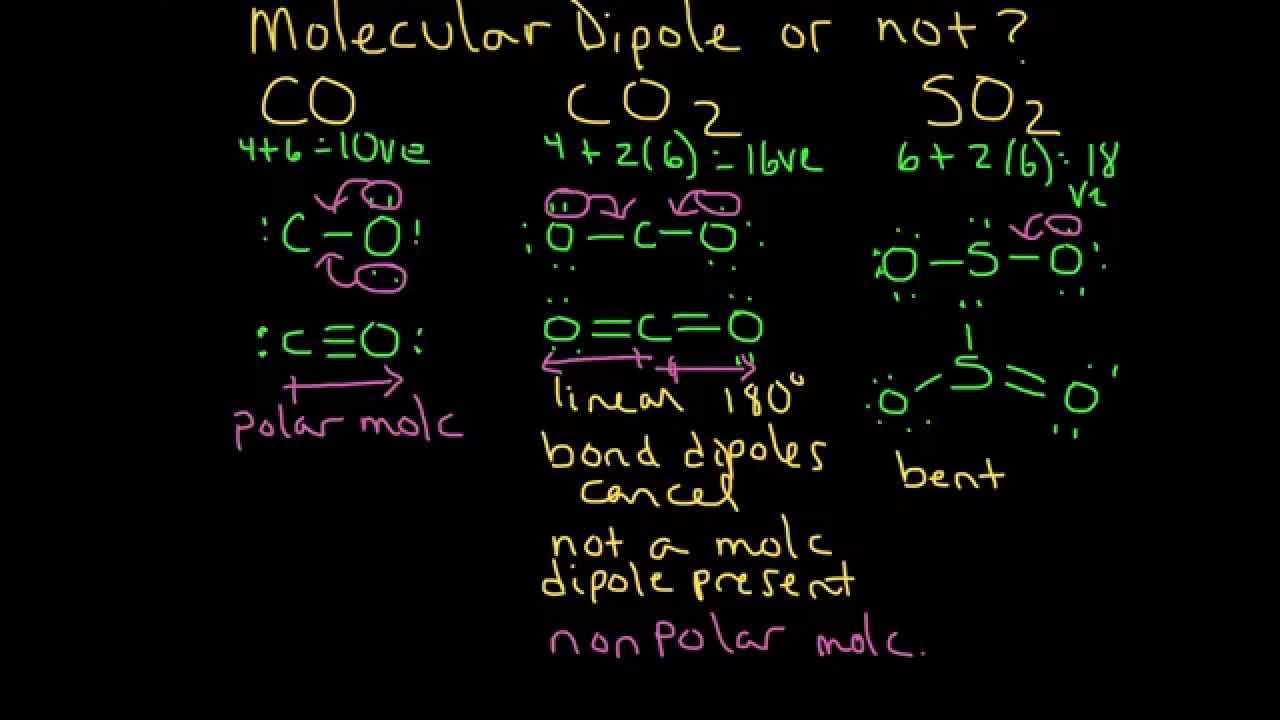
Molecular Dipole Moment Example 1 Co Co2 And So2 Youtube

Identify The Intermolecular Forces Present In Each Of These Substances H2o Co2 Hci Co Homeworklib
0 Comments